Changing from an unusual high-temperature demixing to a UCST-type in mixtures of 1-alkyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}amide and arenes†‡
Abstract
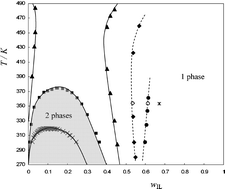
Upper critical solution temperature (UCST) combined with a high-temperature demixing type of phase diagrams are reported for binary liquid mixtures containing the ionic liquids, 1-alkyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}amide ([Cnmim][NTf2], where n = 2, 4, 6, 8, 10) and benzene, toluene, or α-methylstyrene (PhC(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), at atmospheric and moderately high pressure. The phase diagrams were determined either using a dynamic method with visual detection of phase transitions or a laser light scattering technique. In our investigations, as the imidazolium alkyl chain length of the ionic liquid increases, the mixtures containing an arene evolve from a rarely found high-temperature demixing behaviour, to “hour-glass”, to the common phase splitting as temperature diminishes (UCST, whenever a critical point was found). For the three systems containing specifically [C10mim][NTf2] with benzene, toluene, or α-methylstyrene, data were also collected up to 5 MPa using a high-pressure laser light scattering apparatus. For all phase diagrams, the critical compositions correspond to low concentrations of the ionic liquid. This fact underlies the possibility that ionic liquids, even in relatively dilute solutions, tend to form multiple-ion aggregates. This was corroborated by electro-spray mass spectrometry.
CH2), at atmospheric and moderately high pressure. The phase diagrams were determined either using a dynamic method with visual detection of phase transitions or a laser light scattering technique. In our investigations, as the imidazolium alkyl chain length of the ionic liquid increases, the mixtures containing an arene evolve from a rarely found high-temperature demixing behaviour, to “hour-glass”, to the common phase splitting as temperature diminishes (UCST, whenever a critical point was found). For the three systems containing specifically [C10mim][NTf2] with benzene, toluene, or α-methylstyrene, data were also collected up to 5 MPa using a high-pressure laser light scattering apparatus. For all phase diagrams, the critical compositions correspond to low concentrations of the ionic liquid. This fact underlies the possibility that ionic liquids, even in relatively dilute solutions, tend to form multiple-ion aggregates. This was corroborated by electro-spray mass spectrometry.

 Please wait while we load your content...
Please wait while we load your content...