1,3,2-Diazaborolyl-functionalized thiophenes and dithiophenes: synthesis, structure, electrochemistry and luminescence†
Abstract
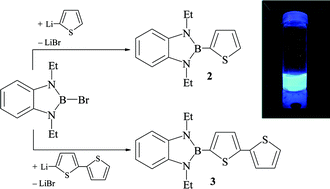
Reaction of 2-bromo-1,3-diethyl-1,3,2-benzodiazaborole (1) with equimolar amounts of thienyl lithium or 2,2′-dithienyl lithium led to the generation of benzodiazaboroles 2 and 3 which are functionalized at the boron atom by a 2-thienyl or a 5-(2,2′-dithienyl) unit. Similarly 2-bromo-1,3-diethyl-1,3,2-naphthodiazaborole (4) and thienyl lithium or 2,2′-dithienyl lithium afforded the naphthoborolyl-substituted thiophene 5 or dithiophene 6. Treatment of 2,5-bis(dibromoboryl)-thiophene 7 with 2 eq. of tBuN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH
CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NtBu in n-hexane followed by sodium amalgam reduction of the obtained bis(diazaborolium) salt 8 gave the 2,5-bis(diazaborolyl)thiophene 9. The 2,5-bis(diazaborolidinyl)-thiophene 10 resulted from the cyclocondensation of 7 with 2 eq. of N,N′-di-tert-butylethylenediamine in the presence of NEt3. Analogously, cyclocondensation of 7 with N,N′-diethylphenylenediamine gave the bis(benzodiazaborolyl) functionalized thiophene 11. The novel compounds were characterized by elemental analysis and spectroscopy (1H-, 11B-, 13C-NMR, MS and UV-VIS). The molecular structure of 3 was elucidated by X-ray diffraction. Cyclovoltammograms show an irreversible oxidation wave at 298–598 vs. Fc/Fc+. The borolylated thiophenes and dithienyls show intense blue luminescence with Stokes shifts of 30–107 nm.
NtBu in n-hexane followed by sodium amalgam reduction of the obtained bis(diazaborolium) salt 8 gave the 2,5-bis(diazaborolyl)thiophene 9. The 2,5-bis(diazaborolidinyl)-thiophene 10 resulted from the cyclocondensation of 7 with 2 eq. of N,N′-di-tert-butylethylenediamine in the presence of NEt3. Analogously, cyclocondensation of 7 with N,N′-diethylphenylenediamine gave the bis(benzodiazaborolyl) functionalized thiophene 11. The novel compounds were characterized by elemental analysis and spectroscopy (1H-, 11B-, 13C-NMR, MS and UV-VIS). The molecular structure of 3 was elucidated by X-ray diffraction. Cyclovoltammograms show an irreversible oxidation wave at 298–598 vs. Fc/Fc+. The borolylated thiophenes and dithienyls show intense blue luminescence with Stokes shifts of 30–107 nm.

 Please wait while we load your content...
Please wait while we load your content...