Oxygen insertion in a carbon–phosphorus bond of the phenylethynyl-di-(tert-butyl)-phosphine bridged dicobalt complex: exploring the nature of oxygen migration using DFT†
Abstract
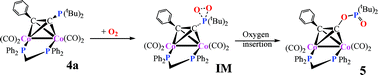
In the process of isolation under aerobic conditions phenylethynyl-di-(tert-butyl)-phosphine bridged dicobalt complex [(µ-PPh2CH2PPh2)Co2(CO)4(µ,η-PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CP(t-Bu)2)] 4a underwent a partial oxidation. The identity of the oxidized product, [(µ-PPh2CH2PPh2)Co2(CO)4(µ,η-PhC
CP(t-Bu)2)] 4a underwent a partial oxidation. The identity of the oxidized product, [(µ-PPh2CH2PPh2)Co2(CO)4(µ,η-PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–O–P(
C–O–P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(t-Bu)2)] 5, was established by spectroscopic means as well as the single-crystal X-ray diffraction method. This is the first crystallographic evidence that unambiguously supports the formation of an organometallic version of a phosphinate ester. The mechanism for the formation of 5 from 4a was proposed, and its validity was examined by DFT means. For the purpose of comparison, a similar mechanism illustrating the transformation of PhC
O)(t-Bu)2)] 5, was established by spectroscopic means as well as the single-crystal X-ray diffraction method. This is the first crystallographic evidence that unambiguously supports the formation of an organometallic version of a phosphinate ester. The mechanism for the formation of 5 from 4a was proposed, and its validity was examined by DFT means. For the purpose of comparison, a similar mechanism illustrating the transformation of PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CP(t-Bu)21O into PhC
CP(t-Bu)21O into PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–O–P(
C–O–P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(t-Bu)25O, the organic counterpart of 5, was examined by the same method. It was found that the metal fragment is indeed capable of assisting the oxidation process by lowering the activation energy, although the effect is small. The impact of the presence of an electron-withdrawing substituent such as a fluorine atom in the alkynylphosphine was also investigated. Results demonstrated that the conversion of fluorine-substituted
O)(t-Bu)25O, the organic counterpart of 5, was examined by the same method. It was found that the metal fragment is indeed capable of assisting the oxidation process by lowering the activation energy, although the effect is small. The impact of the presence of an electron-withdrawing substituent such as a fluorine atom in the alkynylphosphine was also investigated. Results demonstrated that the conversion of fluorine-substituted

 Please wait while we load your content...
Please wait while we load your content...