Direct synthesis of (imine)platinum(ii) complexes by iminoacylation of ketoximes with activated organonitrile ligands
Abstract
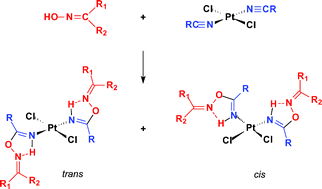
The metal-mediated iminoacylation of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH (1a R1 = R2 = Me; 1b R1 = Me, R2 = Et; 1c R1R2 = C4H8; 1d R1R2 = C5H10) upon treatment with the platinum(II) complex trans-[PtCl2(NCCH2CO2Me)2] 2a with an organonitrile bearing an acceptor group proceeds under mild conditions in dry CH2Cl2 to give the trans-[PtCl2{NH
NOH (1a R1 = R2 = Me; 1b R1 = Me, R2 = Et; 1c R1R2 = C4H8; 1d R1R2 = C5H10) upon treatment with the platinum(II) complex trans-[PtCl2(NCCH2CO2Me)2] 2a with an organonitrile bearing an acceptor group proceeds under mild conditions in dry CH2Cl2 to give the trans-[PtCl2{NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2CO2Me)ON
C(CH2CO2Me)ON![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR1R2}2] 3a–d isomers in moderate yield. The reaction of those
CR1R2}2] 3a–d isomers in moderate yield. The reaction of those ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2Cl)ON
C(CH2Cl)ON![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR1R2}2] 3e–h and 4e–h in moderate to good yield. These reactions are greatly accelerated by microwave irradiation to give, with higher yields (ca. 75%), the same products which were characterized by
CR1R2}2] 3e–h and 4e–h in moderate to good yield. These reactions are greatly accelerated by microwave irradiation to give, with higher yields (ca. 75%), the same products which were characterized by

 Please wait while we load your content...
Please wait while we load your content...