Tricyclohexylphosphine-cyclopalladated ferrocenylimine complexes: synthesis, crystal structures and application in Suzuki and Heck reactions†
Abstract
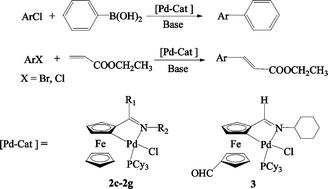
A series of novel tricyclohexylphosphine (PCy3)-cyclopalladated ferrocenylimine complexes 2c–2g have been easily synthesized. These new palladacycles are thermally stable and are not sensitive to air and moisture. Their detailed structures have been determined by single-crystal

 Please wait while we load your content...
Please wait while we load your content...