The reaction of TiF4 with Li(OC(CF3)2Ph): direct synthetic route to the lithium–titanium heterometallic fluoride bridged complex {Li(THF)2TiF3(OC(CF3)2Ph)2}2 and Ti(OC(CF3)2Ph)4 alkoxide†
Abstract
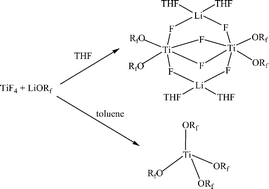
Reaction of TiF4 and LiORf (Rf = C(CF3)2Ph) in the donor solvent-THF lead to the isolation of the heterometallic lithium–titanium complex (THF)2Li(μ-F)2Ti(ORf)2(μ-F)2Ti(ORf)2(μ-F)2Li(THF)2 (1) and Ti(ORf)4 alkoxide (2). Interaction of TiF4 and LiORf in the non-coordinating and low polar

 Please wait while we load your content...
Please wait while we load your content...