Design, synthesis and photophysical studies of an emissive, europium based, sensor for zinc
Abstract
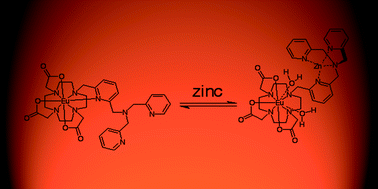
The synthesis of the probe complex Eu·L and photophysical studies in aqueous media are described. Eu·L is designed to be luminescently responsive to ZnII in the presence of a competitive ionic mixture at pH 7.4. The structure of the complex is based upon a carboxylate functionalised

 Please wait while we load your content...
Please wait while we load your content...