Structure of sodium bis(N-methyl-iminodiacetato)iron(iii): trans-meridional N-coordination in the solid state and in solution†
Abstract
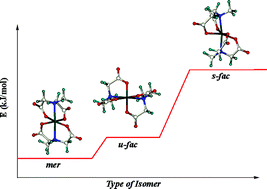
The results of a detailed solid state and solution structural study of the FeIII bis-mida complex [FeIII(mida)2]− (mida = N-methyl-iminodiacetate) are reported. The structure of the sodium salt Na[Fe(mida)2][NaClO4]2·3H2O (1) was determined by single-crystal X-ray analysis. The complex anion in 1 contains a six-coordinate FeIII centre bound to two tridentate mida ligands arranged in the meridional configuration, and the mer FeIIIN2O4 chromophore shows a high degree of distortion from regular octahedral symmetry. Raman- and UV/VIS/NIR spectroscopic measurements showed that no gross changes take place in the FeIII coordination sphere upon redissolution in water. Quantum chemical calculations of all three possible configurations of the [Fe(mida)2]− complex ion in the gas phase support the finding that the mer isomer is more stable than the u-fac (cis) and s-fac (trans) isomers. Redox potential measurements of the FeIII/II(mida) couple in dependence of pH led to the following values for the equilibrium contants: log βIII101 = 11.98 ± 0.05, log βIII102 = 20.49 ± 0.01, pKIIIa1 OH = 7.81; log βII101 = 6.17 ± 0.01, log βII102 = 11.39 ± 0.01.

 Please wait while we load your content...
Please wait while we load your content...