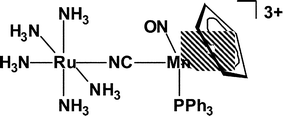
The complexes [(H3N)5RuII(µ-NC)MnILx]2+, prepared from [Ru(OH2)(NH3)5]2+ and [Mn(CN)Lx] {Lx = trans-(CO)2{P(OPh)3}(dppm); cis-(CO)2(PR3)(dppm), R = OEt or OPh; (PR3)(NO)(η-C5H4Me), R = Ph or OPh}, undergo two sequential one-electron oxidations, the first at the ruthenium centre to give [(H3N)5RuIII(µ-NC)MnILx]3+; the osmium(III) analogues [(H3N)5OsIII(µ-NC)MnILx]3+ were prepared directly from [Os(NH3)5(O3SCF3)]2+ and [Mn(CN)Lx]. Cyclic voltammetry and electronic spectroscopy show that the strong solvatochromism of the trications depends on the hydrogen-bond accepting properties of the solvent. Extensive hydrogen bonding is also observed in the crystal structures of [(H3N)5RuIII(µ-NC)MnI(PPh3)(NO)(η-C5H4Me)][PF6]3·2Me2CO·1.5Et2O, [(H3N)5RuIII(µ-NC)MnI(CO)(dppm)2-trans][PF6]3·5Me2CO and [(H3N)5RuIII(µ-NC)MnI(CO)2{P(OEt)3}(dppm)-trans][PF6]3·4Me2CO, between the ammine groups (the H-bond donors) at the Ru(III) site and the oxygen atoms of solvent molecules or the fluorine atoms of the [PF6]− counterions (the H-bond acceptors).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...