Host–guest interaction between cyclen based macrotricyclic ligands and phosphate anions. A potentiometric investigation†
Abstract
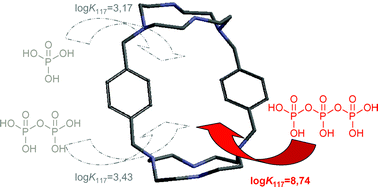
The host–guest interaction between orthophosphate, pyrophosphate and triphosphate anions and three cyclen based macrotricyclic

 Please wait while we load your content...
Please wait while we load your content...