Influence of the stereochemistry of sugars on the selectivity of formation of carbohydrate-derived cyclopentadienyl and indenyl ligands
Abstract
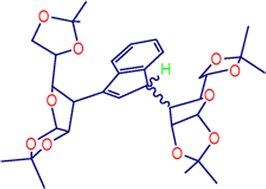
Interaction of lithium cyclopentadienide with a suitable partially protected α-D-allofuranose triflate, 4, epimer at C3 of glucose, gives as a major product, besides the expected glucose-cyclopentadiene, 5, a glucose-disubstituted cyclopentadiene, 6. This unprecedented behaviour, which does not occur with α-D-glucofuranose and other sugars, is tentatively explained by a complexation of LiCp and the oxygen atoms of the isopropylidene function of one molecule of 4 and one of 5, giving a termolecular structure as the result of a template effect. The results of other experiments, such as the use of MgCp2 in place of LiCp or the complexation of oxygen atoms by lithium triflate, which changed the selectivity of the reaction largely in favour of the monosubstitution product 5, support this hypothesis. When lithium indenide is reacted with 4, glucose-monosubstituted and glucose-disubstituted indenes, 8 and 9, respectively, are formed, and 9 is obtained with almost total diastereoselectivity. This result can also be rationalised by a stereoselective complexation of lithium, as shown by separate experiments and by molecular mechanics calculations. Methyltricarbonyl molybdenum(II) complexes have been synthesised and characterised from glucose-monosubstituted 5 and glucose-disubstituted 6 cyclopentadienes.

 Please wait while we load your content...
Please wait while we load your content...