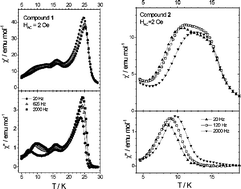
Two new ferromagnetic organic–inorganic hybrid materials {CoII3(H2O)6(pyz)3[WV(CN)8]2}·3.5H2O (1) and {CoII3(H2O)4(4,4′-bpy)3[WV(CN)8]2}·1.5(4,4′-bpy)·6H2O (2) have been synthesised and characterised. The structure of the compounds have been investigated combining EXAFS (extended X-ray absorption fine structure), ES-MS (electrospray mass spectrometry), IR (infrared spectroscopy), UV-VIS electronic spectroscopy and TGA (thermogravimetric analysis) coupled with QMS (quadrupole mass spectrometer) experiments. The studies reveal that both compounds consist of CoII–NC–WV and CoII–L–CoII linkages (L = pyrazine (1) or 4,4′-bipyridine (2)). Both networks are created by cyano-bridged CoII3WV2 chains joined by organic linkers into a 2D architecture. A difference of cobalt coordination numbers in both compounds derived from EXAFS study is consistent with the ES-MS conclusion. The ac magnetic characterisation exhibits the transition to the ferromagnetic phase at TC = 26 K (1) and to the spin glass-like phase at TG = 16 K (2). The frequency dependent χ′(T) and χ″(T) signals indicate the presence of some disorder in spin alignment below ordering temperatures. Both networks are also characterised a by magnetic hysteresis loop of coercive field Hc = 750 Oe (1) and 1200 Oe (2) at T = 4.2 K.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...