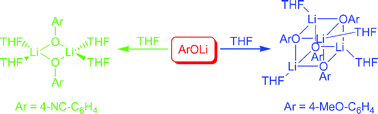
The para-substituted lithium aryloxides [{4-NC–C6H4OLi·(Pyr)2}2·Pyr] 1a, [{4-NC–C6H4OLi·(THF)2}2] 1b, [{4-MeO–C6H4OLi·Pyr}4] 2a, [4-MeO–C6H4OLi·(THF)n] 2b, [{4-NC-2,6-(t-Bu)2–C6H2OLi·(Pyr)2}∞] 3a, [{4-NC-2,6-(t-Bu)2–C6H2OLi·(THF)2}∞] 3b, [{4-MeO-2,6-(t-Bu)2–C6H2OLi·Pyr}2·(Pyr)2] 4a, and [4-MeO-2,6-(t-Bu)2–C6H2OLi·(THF)n] 4b were prepared by the direct deprotonation of the corresponding phenol with an alkyllithium base (BuLi or MeLi) in the appropriate solvent, either pyridine or THF. All compounds were characterized by 1H and 13C NMR spectroscopy, and the crystal structures of 1a, 1b, 2a, 3a, 3b and 4a were elucidated. The cyano derivatives 1a and 1b adopt discrete tetrasolvated Li2O2 ring dimers whereas the methoxy analogue 2a crystallizes as a tetrasolvated molecular tetramer with a pseudo cubic Li4O4 core. The sterically encumbered cyano derivatives 3a and 3b form isostructural 1D polymeric chains of monomers via bridging of the phenolate ligands through Li⋯NC and Li–O contacts. In comparison, the crystal structure of the methoxy counterpart 4a is a disolvated molecular Li2O2 ring dimer. Solution NMR spectroscopic studies of 1–4 in d5-pyridine and d8-THF indicate that the methoxy complexes are more highly aggregated than the cyano derivatives, consistent with the solid-state studies. Ab initio molecular orbital calculations at the HF/6-31G* level of theory indicate that the origin of the aggregation state variations between the cyano and methoxy complexes is due to electronic effects.

 Please wait while we load your content...
Please wait while we load your content...