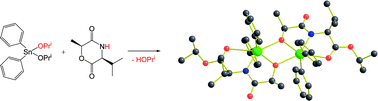
(3S,6S)-3-Isopropyl-6-methyl-morpholine-2,5-dione (1), and (3S,6S)-3,6-dimethyl-morpholine-2,5-dione (2), do not enter into ring-opening polymerization reactions with metal catalyst precursors commonly employed for lactides, and with Sn(II) octanoate, only low molecular weight oligomers are obtained. Reactions with R2SnX2 compounds, where R = Ph, Bun and p-Me2NC6H4 and X = OPri or NMe2, reveal that ring-opening of the morpholine-2,5-diones does occur, but that polymerization is terminated by the formation of kinetically-inert products such as {Ph2Sn[μ,η3-OCH(Me)CONCH(Pri)COOPri]}2 (3), and {[Bun)2Sn[μ,η3-OCH(Me)CONCH(Me)CONMe2]}2 (4), with elimination of HX. Ph3SnOPri is seen to react reversibly with morpholine-2,5-diones in toluene-d8 by 1H NMR spectroscopy while (Bun)3SnNMe2 reacts by ring opening to give (Bun)3SnOCH(Me)C(O)NHCHMeC(O)NMe2. The new organotin compounds have been characterized by 1H, 13C{1H} and 118Sn NMR spectroscopy and compounds 1, 2, 3 and 4 by single crystal X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...