Stabilization of the tautomers HP(OH)2 and P(OH)3 of hypophosphorous and phosphorous acids as ligands
Abstract
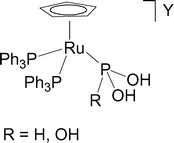
Treatment of [CpRu(PPh3)2Cl] 1 with the stoichiometric amount of H3PO2 or H3PO3 in the presence of chloride scavengers (AgCF3SO3 or TlPF6) yields compounds of formula [CpRu(PPh3)2(HP(OH)2)]Y (Y = CF3SO32a or PF62b) and [CpRu(PPh3)2(P(OH)3)]Y (Y = CF3SO33a or PF63b) which contain, respectively, the HP(OH)2 and P(OH)3 tautomers of hypophosphorous and phosphorous acids bound to ruthenium through the phosphorus atom. The triflate derivatives 2a and 3a react further with hypophosphorous or phosphorous acids to yield, respectively, the complexes [CpRu(PPh3)(HP(OH)2)2]CF3SO34 and [CpRu(PPh3)(P(OH)3)2]CF3SO35 which are formed by substitution of one molecule of the acid for a coordinated triphenylphosphine molecule. The compounds 2 and 3 are quite stable in the solid state and in solutions of common organic solvents, but the hexafluorophosphate derivatives undergo easy transformations in CH2Cl2: the hypophosphorous acid complex 2b yields the compound [CpRu(PPh3)2(HP(OH)2)]PF2O26, whose difluorophosphate anion originates from hydrolysis of PF6−; the phosphorous acid complex 3b yields the compound [CpRu(PPh3)2(PF(OH)2)]PF2O27, which is produced by hydrolysis of hexafluorophosphate and substitution of a fluorine for an OH group of the coordinated acid molecule. All the compounds have been characterized by elemental analyses and NMR measurements. The crystal structures of 2a, 3a and 7 have been determined by X-ray diffraction methods.

 Please wait while we load your content...
Please wait while we load your content...