Size matters: why nanomaterials are different
Abstract
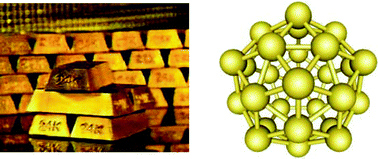
Gold is known as a shiny, yellow noble metal that does not tarnish, has a face centred cubic structure, is non-magnetic and melts at 1336 K. However, a small sample of the same gold is quite different, providing it is tiny enough: 10 nm particles absorb green light and thus appear red. The meltingtemperature decreases dramatically as the size goes down. Moreover, gold ceases to be noble, and 2–3 nm

 Please wait while we load your content...
Please wait while we load your content...