The oxidation of organocuprates—an offbeat strategy for synthesis
Abstract
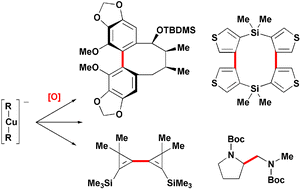
The creation of carbon–carbon and carbon–heteroatom bonds by the oxidation of organocuprates is useful in a range of challenging contexts including the formation of biaryl bonds within medium rings, diene synthesis and the coupling of tertiary carbon centres. This tutorial review introduces these applications and recent developments which have served to improve its functional group tolerance and its utility in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...