Kinetics of the reactions of CH2Br and CH2I radicals with molecular oxygen at atmospheric temperatures
Abstract
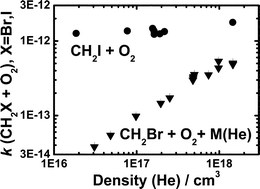
The kinetics of the reactions of CH2Br and CH2I radicals with O2 have been studied in direct measurements using a tubular flow reactor coupled to a photoionization mass spectrometer. The radicals have been homogeneously generated by pulsed laser photolysis of appropriate precursors at 193 or 248 nm. Decays of radical concentrations have been monitored in time-resolved measurements to obtain the reaction rate coefficients under pseudo-first-order conditions with the amount of O2 being in large excess over radical concentrations. No

 Please wait while we load your content...
Please wait while we load your content...