Abstract
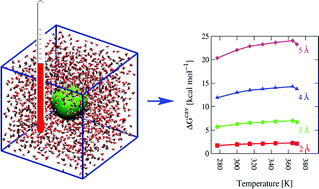
A high level polarizable force field is used to study the temperature dependence of hydrophobic hydration of small-sized molecules from computer simulations. Molecular dynamics (MD) simulations of liquid
a
Indian Institute of Technology, Delhi, Department of Chemical Engineering, Hauz Khas, New Delhi, India
E-mail:
reema.mahajan@gmail.com
b
Johannes Kepler University of Linz, Institute of Graphics and Parallel Processing, Altenberger Straβe 69, Linz, Austria
E-mail:
kranzlmueller@gup.jku.at; volkert@gup.jku.at
c
Michigan Technological University, Department of Physics, 1400 Townsend Drive, Houghton, MI, USA
E-mail:
hansmann@mtu.edu; shoefing@mtu.edu
d John von Neumann Institute for Computing, FZ Jülich, Jülich, Germany
A high level polarizable force field is used to study the temperature dependence of hydrophobic hydration of small-sized molecules from computer simulations. Molecular dynamics (MD) simulations of liquid

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
R. Mahajan, D. Kranzlmüller, J. Volkert, U. H. E. Hansmann and S. Höfinger, Phys. Chem. Chem. Phys., 2006, 8, 5515 DOI: 10.1039/B611200E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
