In-situUV-visible study of Pd nanocluster formation in solution†
Abstract
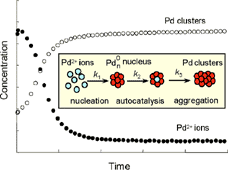
The formation of Pd nanoclusters in solution is studied. This system has two types of light-absorbing species: Pd ions which absorb light via electronic transitions and Pd clusters and aggregates which absorb light via valence–conduction transitions and also scatter light due to their nanometric dimensions. Here we monitor these dynamic changes using

 Please wait while we load your content...
Please wait while we load your content...