Nucleation and growth of cobalt nanostructures on highly oriented pyrolytic graphite
Abstract
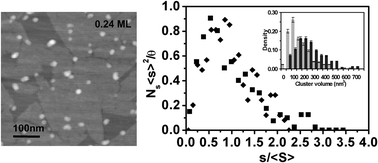
Cobalt in the form of three-dimensional (3D) hemispherical clusters (size ∼10–30 nm) were observed to grow on pristine graphite surfaces via a Volmer–Weber growth mode.

 Please wait while we load your content...
Please wait while we load your content...