Quantum yields for OH production in the photodissociation of HNO3 at 248 and 308 nm and H2O2 at 308 and 320 nm†
Abstract
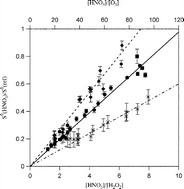
The quantum yields for OH formation from the photolysis of HNO3 were measured to be (0.88 ± 0.09) at 248 and (1.05 ± 0.29) at 308 nm and of H2O2 to be (1.93 ± 0.39) at 308 and (1.96 ± 0.50) at 320 nm. The quoted uncertainties are at the 95% confidence level and include estimated systematic uncertainties. OH radicals were produced using pulsed laser photolysis and monitored using pulsed laser-induced fluorescence. Quantum yields were measured relative to the OH quantum yields from a reference system. The measured quantum yields at 248 nm are in agreement with previous direct determinations. The quantum yield values at 308 and 320 nm are the first direct quantum yield measurements at these wavelengths and confirm the values currently recommended for atmospheric model calculations. Rate coefficients (at 298 K) for the OH + H2O2 and OH + HNO3 + M (in 100 Torr of N2) reactions were measured during this study to be (1.99 ± 0.16) × 10−12 cm3 molecule−1 s−1 and (1.44 ± 0.12) × 10−13 cm3 molecule−1 s−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...