An ab initio study of the electronic structure of BCl 2+3 and its decomposition pathways
Abstract
Ab initio methods have been used to characterise the lowest energy potential energy surfaces of 1BCl2+3 and 3BCl2+3. The methods used are MP2, CCSD, CCSD(T) and MCSCF with 6-311G(d), cc-pVTZ and aug-cc-pVTZ basis sets. While the singlet surface is relatively straight-forward, the triplet surface is very complicated, with many stationary points in close energetic proximity. The singlet surface can fragment to the following products (1BCl +
1Cl+
+
1Cl+), (1Cl+
+
1B+
+
1Cl2), (2BCl+
+
2Cl+2), while the triplet surface can fragment to (1BCl+2
+
3Cl+) and (2BCl2+2
+
2Cl). 2BCl2+2 can further fragment to (1Cl+
+
2BCl+). These results are in good agreement with previous 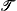 1 diagnostic values have been calculated for all of the stationary points of BCl2+3, using the method of Lee and Taylor. These data, together with CCSD/CCSD(T) energy differences and MCSCF calculations, are used to conclude that most of the stationary points on the singlet surface are well represented using single reference methods. The stationary points of the triplet system have
1 diagnostic values have been calculated for all of the stationary points of BCl2+3, using the method of Lee and Taylor. These data, together with CCSD/CCSD(T) energy differences and MCSCF calculations, are used to conclude that most of the stationary points on the singlet surface are well represented using single reference methods. The stationary points of the triplet system have 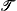 1 diagnostic values greater than those for the singlet system, as expected when using the closed-shell
1 diagnostic values greater than those for the singlet system, as expected when using the closed-shell 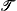 1 diagnostic method of Lee and Taylor. However, all of the structures have acceptable single reference wavefunctions if the open-shell upper limit of Rienstra-Kiracofe et al. (0.045) is used, a conclusion fully supported by CCSD/CCSD(T) energy differences. CCSD(T) energies determined for the fragmentation asymptotes have been compared with
1 diagnostic method of Lee and Taylor. However, all of the structures have acceptable single reference wavefunctions if the open-shell upper limit of Rienstra-Kiracofe et al. (0.045) is used, a conclusion fully supported by CCSD/CCSD(T) energy differences. CCSD(T) energies determined for the fragmentation asymptotes have been compared with


 Please wait while we load your content...
Please wait while we load your content...