Chlorofluoroiodomethane as a potential candidate for parity violation measurements
Abstract
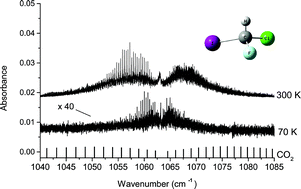
CHFClI is among the more favorable molecules for parity violation (PV) measurements in molecules. Despite the fact that calculated PV effects are two orders of magnitude smaller than in some

 Please wait while we load your content...
Please wait while we load your content...