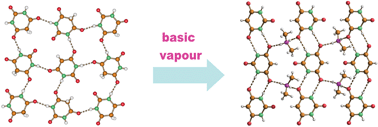
The two known crystalline polymorphic forms of barbituric acid (C4H4N2O3) (form I and form II) and the dihydrate form [(C4H4N2O3)·2H2O] have been reacted with vapours of ammonia, methylamine, and dimethylamine and the crystalline products investigated by means of single crystal and powder X-ray diffraction, thermogravimetric analysis, differential scanning calorimetry and infrared spectroscopy. It has been shown that form I, II and the dihydrate form of barbituric acid react with ammonia leading to the same crystalline ammonium barbiturate salt NH4(C4H3N2O3) 1a, while the gas–solid reactions of form II with methylamine, and dimethylamine yield the corresponding crystalline salts, CH3NH3(C4H3N2O3) 1b and (CH3)2NH2(C4H3N2O3) 1c. Thermal desorption of the bases at ca. 200 °C leads to formation of a new crystal form of barbituric acid, form III, as confirmed by H1-NMR spectroscopy and by chemical behaviour. De-hydration of the dihydrate form has also been investigated showing that it releases water to yield exclusively crystals of form II.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...