Structure and rheology of aqueous micellar solutions and gels formed from an associative poly(oxybutylene)–poly(oxyethylene)–poly(oxybutylene) triblock copolymer
Abstract
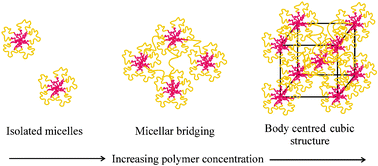
The structure and shear flow behaviour of aqueous micellar solutions and gels formed by an amphiphilic poly(oxybutylene)–poly(oxyethylene)–poly(oxybutylene)

 Please wait while we load your content...
Please wait while we load your content...