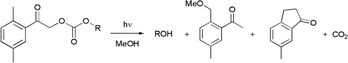
Synthesis and photochemistry of a photochemically removable protecting group for alcohols and phenols, based on the 2,5-dimethylphenacyl (DMP) chromophore, is described. DMP carbonates release the corresponding hydroxy group containing molecules in high isolated yields (>70%), with quantum yields Φ
= 0.1–0.2 in methanol and Φ
= 0.36–0.51 in cyclohexane. The reactions proceed predominantly by the triplet pathway via E-photoenols, the lifetimes of which of approximately 2 s or 3 ms in cyclohexane or methanol, respectively, were determined by laser flash photolysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...