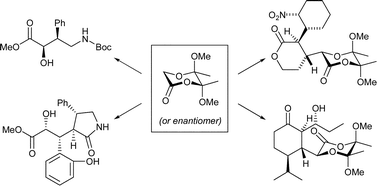
Michael, Michael–aldol and Michael–Michael reactions of enolate equivalents of butane-2,3-diacetal protected glycolic acid derivatives
Abstract
Consecutive coupling reactions of butane-2,3-diacetal protected glycolic acid derivatives with Michael acceptors and

 Please wait while we load your content...
Please wait while we load your content...