A diversity oriented four-component approach to tetrahydro-β-carbolines initiated by Sonogashira coupling
Abstract
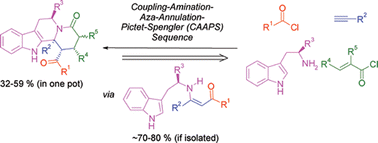
A consecutive four-component synthesis of highly-substituted tetrahydro-β-carbolines 6 can be achieved by a coupling-aminatio-aza-annulation-Pictet–Spengler (CAAPS) sequence creating five new σ-bonds and four new stereocenters in a one-pot fashion. The structures were unambiguously supported by X-ray structure analyses.

 Please wait while we load your content...
Please wait while we load your content...