Synthesis of a non-hydrolyzable estrone sulfate analogue bearing the difluoromethanesulfonamide group and its evaluation as a steroid sulfatase inhibitor
Abstract
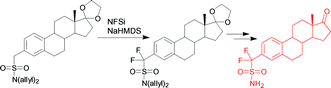
Steroid sulfatase (STS) catalyzes the hydrolyis of steroidal sulfates such as estrone sulfate (ES1) and is considered to be an attractive target in the treatment of steroid dependent cancers. A non-hydrolyzable estrone sulfate (ES1) analogue bearing an α,α-difluorosulfonamide moiety at the 3-position on the A-ring, compound 7, was synthesized. Key to the success of this synthesis was the first use of the allyl group as a sulfonamide protecting group. The pKa of this ES1 mimic in 0.1 M bis-tris propane, 10% DMSO was determined to be 8.05 using 19F NMR. Compound 7 is a reversible inhibitor with a Ki similar to that of its sulfonate analogue at pH 7.0. It is more potent than its nonfluorinated sulfonamide analogue and, its inhibitory potency increases with increasing pH, a trend opposite to that of other STS inhibitors. Possible reasons for this are presented.

 Please wait while we load your content...
Please wait while we load your content...