The one-pot, multi-component construction of highly substituted tetrahydropyran-4-ones using the Maitland–Japp reaction†‡
Abstract
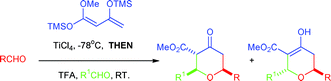
A one-pot, multi-component reaction for the synthesis of highly substituted tetrahydropyran-4-ones, based on the long forgotten Maitland–Japp reaction has been realised. Two different

 Please wait while we load your content...
Please wait while we load your content...