Stereochemistry of reactions of the inhibitor/substrates l- and d-β-chloroalanine with β-mercaptoethanol catalysed by l-aspartate aminotransferase and d-amino acid aminotransferase respectively†
Abstract
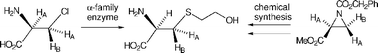
Two members of the α-family of PLP-dependent enzymes, L-aspartate aminotransferase and D-amino acid aminotransferase, have been shown to catalyse β-substitution of L- and D-β-chloroalanine respectively with β-mercaptoethanol, reactions typical of the β-family of PLP-dependent enzymes. The reaction catalysed by L-aspartate aminotransferase has been shown to occur with retention of stereochemistry, a typical outcome for reactions catalysed by β-family enzymes. There are also indications that the reaction catalysed by D-amino acid aminotransferase may involve retention of stereochemistry. Both enzymes have been shown to catalyse exchange at C-3 when the appropriate enantiomer of β-chloroalanine is the substrate.

 Please wait while we load your content...
Please wait while we load your content...