Abstract
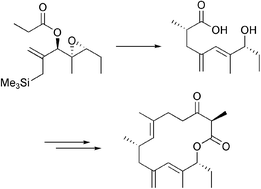
The construction of the fourteen membered ring present in galbonolide B 1 is reported. The 10,11-diene system present in the southern portion of 1 has been constructed using an ester enolate rearrangement/silicon mediated fragmentation cascade, whilst the

 Please wait while we load your content...
Please wait while we load your content...