Near IR emitting isothiocyanato-substituted fluorophores: their synthesis and bioconjugation to monoclonal antibodies
Abstract
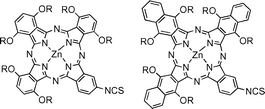
Two near IR emitting fluorophores, based on the phthalocyanine and naphthalocyanine chromophores, which also bear a single isothiocyanato group suitable for conjugation to proteins are reported; their utility as luminescent probes is demonstrated by conjugation to monoclonal antibodies and the ability of these conjugates to selectively bind cells bearing the relevant antigen.

 Please wait while we load your content...
Please wait while we load your content...