Synthesis and characterization of a novel functionalized azanonaborane cluster for boron neutron capture therapy
Abstract
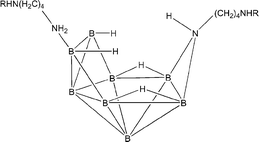
The reactivity of an azanonaborane cluster containing free amino groups {H2N(CH2)4H2NB8H11NH(CH2)4NH2} towards ketones and aldehydes is investigated. In a one step reaction, the reductive amination of some ketones and aldehydes (namely acetone, benzaldehyde, 3-hydroxybenzaldehyde, 4-hydroxybenzaldehyde, 4-nitrobenzaldehyde, 4-acetoxybenzaldehyde, and 4-acetamidobenzaldehyde) with an azanonaborane cluster in the presence of H3BNH2(CH2)4NH2 gives monoalkylamino derivatives of the azanonaborane cluster {RHN(CH2)4H2NB8H11NH(CH2)4NHR} where (R = (Me)2CH–, C6H5CH2–, 3-OHC6H4CH2–, 4-OHC6H4CH2–, 4-NO2C6H4CH2–, 4-MeOCOC6H4CH2–, or 4-NH2COC6H4CH2-). The functionalized derivatives of the {B8N} cluster can be used in boron neutron capture therapy for tumors (BNCT). Similarly, the reductive amination of 5-(4′-formylphenyl)-10,15,20-triphenylporphyrin with the {B8N} cluster gave a porphyrin bearing azanonaborane cluster, while a porphyrin dimer linked by an azanonaborane moiety was obtained following the same method, starting with a 2 : 1 molar ratio of porphyrin : {B8N} cluster. 5,10,15,20-Tetraformylphenylporphyrin gave the chance to increase the percentage of boron in the resulting boronated porphyrin, which is considered an important factor for a BNCT delivery agent. With these compounds, the cell toxicity using V79 cells was carried out to determine whether these compounds would have favorable biological properties.

 Please wait while we load your content...
Please wait while we load your content...