Factors affecting the selection of products from a photochemically generated singlet biradical
Abstract
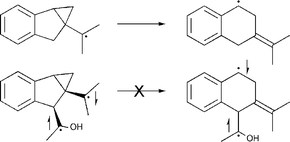
The chemistries of a monoradical of the ultrafast “radical-clock” type and a structurally related singlet biradical, generated by Norrish type II photochemistry, are compared. The monoradical is found to undergo the characteristic ring-opening reaction of its class at about 1010 s−1 at room temperature. However, the singlet biradical shows no evidence of the analogous ring-opening reaction. The contrasting chemistry is traced not to a fundamental difference in electronic structure of the two intermediates, but rather to a steric interaction that the biradical alone would have to suffer during the ring opening. Although the magnitude of the steric hindrance is small (estimated 15–20 kJ mol−1), it is enough to shut down the reaction, because the biradical has other facile product-forming reactions available.

 Please wait while we load your content...
Please wait while we load your content...