Enantioselective synthesis of erythro-4-deoxyglycals as scaffolds for target- and diversity-oriented synthesis: new insights into glycal reactivity†
Abstract
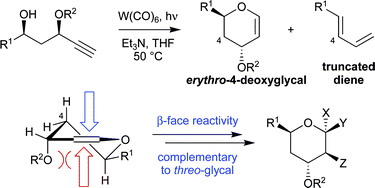
An efficient, enantioselective synthesis of erythro-4-deoxyglycals has been developed using asymmetric

 Please wait while we load your content...
Please wait while we load your content...