Novel carbamate derivatives of 4-β-amino-4′-O-demethyl-4-desoxypodophyllotoxin as inhibitors of topoisomerase II: synthesis and biological evaluation
Abstract
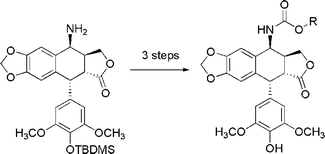
A novel series of carbamate derivatives of 4-β-amino-4′-O-demethyl-4-desoxypodophyllotoxin were synthesized. Their effect on human DNA topoisomerase II and antiproliferative activity was evaluated. Compounds 4a–c, 4g, 4j and 4k are topoisomerase II poisons that induce double-stranded breaks in DNA and exhibit increased cytotoxicity compared to etoposide.

 Please wait while we load your content...
Please wait while we load your content...