3-Aryl β-carbolin-1-ones as a new class of potent inhibitors of tumor cell proliferation: synthesis and biological evaluation
Abstract
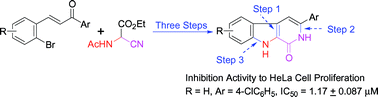
A novel three-step synthesis of 3-aryl β-carbolin-1-ones from non-indole starting materials has been developed. The two nitrogen atoms in β-carbolin-1-one were introduced efficiently by Michael addition of ethyl acetamidocyanoacetate to chalcone. The desired pyridone and indole rings were assembled by an intramolecular ketone–nitrile annulation mediated by aqueous HCl–HOAc and a Cu(I)-catalyzed intramolecular N-arylation of the amide, respectively. The target compounds were found to possess significant activity against tumor cell proliferaton.

 Please wait while we load your content...
Please wait while we load your content...