Natural products to drugs: daptomycin and related lipopeptide antibiotics
Abstract
Covering: 1953 to September 2005
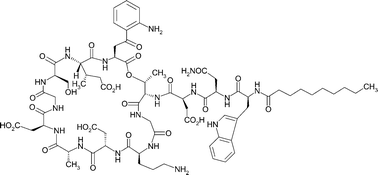
Daptomycin (Cubicin®) is a lipopeptide antibiotic approved in the USA in 2003 for the treatment of skin and skin structure infections caused by Gram-positive pathogens. It is a member of the 10-membered cyclic lipopeptide family of antibiotics that includes A54145, calcium-dependent antibiotic (CDA), amphomycin, friulimicin, laspartomycin, and others. This review highlights research on this class of antibiotics from 1953 to 2005, focusing on more recent studies with particular emphasis on the interplay between structural features and antibacterial activities; chemical modifications to improve activity; the genetic organization and biosynthesis of lipopeptides; and the genetic engineering of the daptomycin biosynthetic pathway to produce novel derivatives for further chemical modification to develop candidates for clinical evaluation.

 Please wait while we load your content...
Please wait while we load your content...