Novel supramolecular charge-transfer systems based on bis(18-crown-6)stilbene and viologen analogues bearing two ammonioalkyl groups†
Abstract
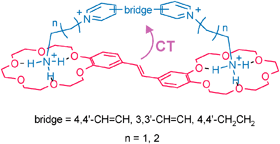
A series of new viologen analogues bearing two ammonioalkyl groups (2–4) were synthesized in order to study their complexation with bis(18-crown-6)stilbene (1b).

 Please wait while we load your content...
Please wait while we load your content...