Synthesis and interfacial properties of amphiphatic cryptophanes†
Abstract
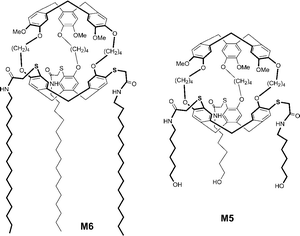
The new amphiphatic cryptophanes M5 and M6 were synthesized from their precursor M4. They present interesting properties due to their long chain substituents on one of the cyclotriveratrylene moieties. M4 was synthesized from a thio-cyclotriveratrylene platform obtained from

 Please wait while we load your content...
Please wait while we load your content...