A study of the effects of subunit pre-orientation for diarylpyrrole esters; design of new aryl-heteroaryl fluorescent sensors
Abstract
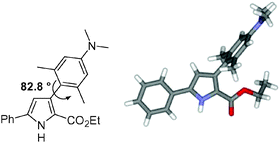
The synthesis, single crystal X-ray structures, spectroscopic properties and molecular mechanics calculations of three systematically substituted 3,5-diaryl-1H-pyrrole-2-carboxylic acid ethyl esters 1a–c are described. The goal is to develop design principles for the generation of new class of fluorescent switches constructed from an aryl-heteroaryl architecture containing a virtual (C0) spacer. The spectroscopic effects of the

 Please wait while we load your content...
Please wait while we load your content...