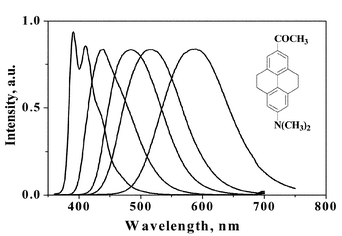
The tetrahydropyrene derivatives 2-N,N-dimethylamino-7-nitro-4,5,9,10-tetrahydropyrene (1) and 2-N,N-dimethylamino-7-acetyl-4,5,9,10-tetrahydropyrene (2) were synthesized and characterized. Photophysical properties of these molecules were investigated in several solvents. The absorption spectrum of 1 shows a slight red shift with solvent polarity, whereas that of 2 remains more or less unchanged. Fluorescence spectra of these compounds exhibit large, solvent-polarity-dependent Stokes shifts. The Stokes shifts are correlated to ET(30) and ENT parameters and were quantitatively analyzed by the Mataga–Liptay equation. Both compounds show low fluorescence quantum yields in cyclohexane. Nanosecond flash photolysis studies suggested that the low quantum yield in cyclohexane is due to intersystem crossing to a triplet state. In the case of 2, the fluorescence quantum yields are high in all other solvents. In the case of 1 fluorescence quantum yields are very low in polar solvents and this is explained by invoking a twisted intramolecular charge transfer state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...