Oxidised guanidinohydantoin (Ghox) and spiroiminodihydantoin (Sp) are major products of iron- and copper-mediated 8-oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanosine oxidation
Abstract
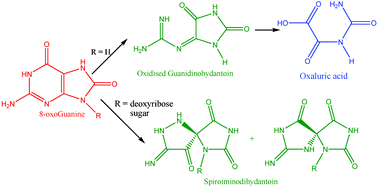
8-Oxo-7,8-dihydroguanine (8-oxoGua), an important biomarker of DNA damage in oxidatively generated stress, is highly reactive towards further oxidation. Much work has been carried out to investigate the oxidation products of 8-oxoGua by one-electron oxidants, singlet oxygen, and peroxynitrite. This report details for the first time, the iron- and copper-mediated Fenton oxidation of 8-oxoGua and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo). Oxidised guanidinohydantoin (Ghox) was detected as the major product of oxidation of 8-oxoGua with iron or copper and hydrogen peroxide, both at pH 7 and pH 11. Oxaluric acid was identified as a final product of 8-oxoGua oxidation. 8-oxodGuo was subjected to oxidation under the same conditions as 8-oxoGua. However, dGhox was not generated. Instead, spiroiminodihydantoin (Sp) was detected as the major product for both iron and copper mediated oxidation at pH 7. It was proposed that the oxidation of 8-oxoGua was initiated by its one-electron oxidation by the metal species, which leads to the reactive intermediate 8-oxoGua˙+, which readily undergoes further oxidation. The product of 8-oxoGua and 8-oxodGuo oxidation was determined by the 2′-deoxyribose moiety of the 8-oxodGuo, not whether copper or iron was the metal involved in the oxidation.

 Please wait while we load your content...
Please wait while we load your content...