A novel organometallic route to phenylethenyl-modified polysiloxanes
Abstract
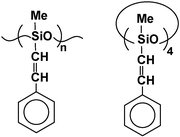
We have synthesized a series of cyclic and linear siloxane materials with phenylethenyl substituents via transition metal complex-catalyzed coupling of the respective vinylsiloxane systems with styrene and α-methylstyrene. It has been shown that the non-carbene metal catalysts [RuCl(H)(CO)(PPh3)3] and [RuCl(SiMe3)(CO)(PPh3)2] are the most effective ones, pointing to a silylative coupling pathway as the most plausible mechanistic route. The process was studied in the presence of a series of catalysts and styrene polymerization inhibitors under different reaction conditions, leading to useful silicone materials characterized by high refractive index values ranging from 1.51 to 1.59 due to strong π-conjugation in side chain substituents.

 Please wait while we load your content...
Please wait while we load your content...