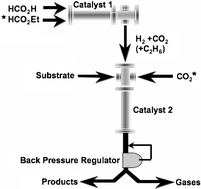
Continuous fixed-bed hydrogenation reactions are one of the most promising reactions studied under supercritical conditions. A reactor and supporting equipment has been developed in a collaboration between the University of Nottingham and HEL Ltd. to provide the means for small-scale experimental research. The high pressure gases required to achieve the supercritical state are not supplied by bottled or liquefied gases, but by the in situ decomposition of formic acid, HCO2H, which can be selectively decomposed to produce CO2 and H2. These gases can be used directly as both the supercritical solvent and reagent gases. Further control of the H2 concentration can be achieved by the parallel decomposition of other liquid precursors, namely ethyl formate HCO2Et, which can produce C2H6
+ CO2. We report the hydrogenation of several organic substrates to demonstrate this approach, its application in research and its potential as a development tool.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...