The first use of [Cr(N)Cl4]2− as a starting material in chromium(V) nitrido chemistry is demonstrated in simple, high yield, metathesis reactions with the pseudohalogens SCN− and N3− yielding five-coordinate, labile complexes: [Cr(N)(NCS)4]2− and [Cr(N)(N3)4]2−, which have been crystallized and characterized by single-crystal X-ray diffraction. Reaction of [Cr(N)(NCS)4]2− with 1,10-phenanthroline furnishes six-coordinate [Cr(N)(NCS)3(phen)]−, wherein phenanthroline coordinates to the position trans to the nitrido ligand. The trans influence of the nitrido ligand leads to a bond length difference of 0.223 Å between the axial and equatorial ligators from the phenanthroline ligand. The absorption band with lowest energy in these pseudo-linear complexes is assigned as the electric dipole forbidden transition dxy→ dx−y based on intensities and its variation with the nature of the equatorial ligators. This absorption provides the spectrochemical series for the equatorial ligands, which is found to be numerically almost identical to that determined for chromium(III). DFT calculations reproduce the observed structures and corroborate the ligand field picture of the electronic structure of these complexes.
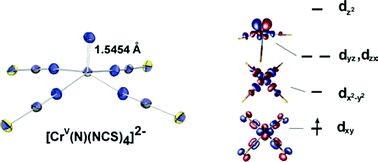
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
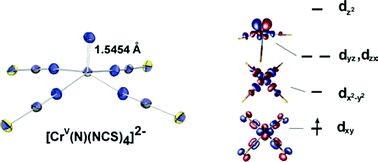

 Please wait while we load your content...
Please wait while we load your content...