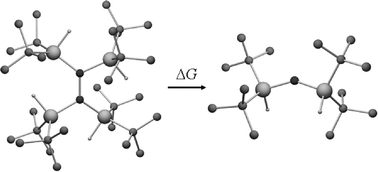
Quantum chemical calculations of the structures and thermodynamics of homolytic dissociation of the central P–P and N–N bonds in tetrakis(disyl)diphosphine and tetrakis(di-tert-butylsilyl)hydrazine have been performed. The theory predicted negative standard enthalpies for homolytic bond dissociation in both cases, −71.0 and −108.4 kJ mol−1 for the diphosphine and hydrazine, respectively, using the ONIOM (MP2/6-31+G*:B3LYP/3-21G*) level. The dissociation is accompanied by considerable structural changes in the radicals as compared to the corresponding fragments of the parent molecules, resulting in low dissociation enthalpies. The most pronounced changes in both radicals are the relaxation of bond angles in the substituents and a conformational change in the orientation of the substituent groups. In addition, the bis(di-tert-butylsilyl)aminyl radical displays a considerable increase in Si–N–Si angle and shortening of the Si–N bonds upon dissociation. These changes are not associated with any appreciable delocalisation of the lone electron, as the spin density is found from the B3LYP/3-21G* calculations to be largely concentrated on the nitrogen atom. It has been also shown that although the dissociation energies are low for both compounds, the intrinsic energies of the central bonds are still high, 140.6 kJ mol−1 for the P–P bond in tetrakis(disyl)diphosphine and 490.6 kJ mol−1 for the N–N bond in tetrakis(di-tert-butylsilyl)hydrazine, using the ONIOM method. The calculations predict that the dissociation of tetrakis(disyl)diphosphine would have negative free energy even without taking relaxation of the fragments into account, while the full potential of releasing about 306 kJ mol−1 of energy stored in the ligands of tetrakis(di-tert-butylsilyl)hydrazine is only fully realised upon a considerable separation of the fragments.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...