Oxygen activation by a macrocyclic chromium complex. Mechanism of hydroperoxo-chromium(iii) to oxo-chromium(v) transformation
Abstract
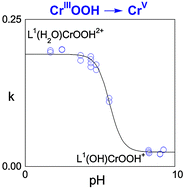
The transformation of the hydroperoxo complex L1(H2O)CrOOH2+ (L1 = 1, 4 8, 11-tetraazacyclotetradecane) to an oxo-chromium(V) species is a first-order process throughout the pH range examined, 1.7 < pH < 9.2. The pH dependence of the rate constant (k1) yielded an apparent pKa of 5.6 for L1(H2O)CrOOH2+. In the acidic range, (pH <4), the value of k1 is 0.191 s−1. At the other extreme, pH >7.5, k1 = 0.025 s−1. No [H+]-dependence is observed within the two limiting regimes, clearly ruling out a simple attack by H+ at the hydroperoxo group. The temperature dependence of k1 in 0.020 M HClO4 yielded the activation parameters ΔH‡ = 53.7 kJ mol−1 and ΔS‡ = −80.5 J mol−1 K−1.

 Please wait while we load your content...
Please wait while we load your content...